Pain relief and deswelling by mobile thermotherapy
The portable mollicool thermotherapy system provides optimal cooling after trauma, surgery, exercise and during rehabilitation independent of freezer infrastructure and electric grid.
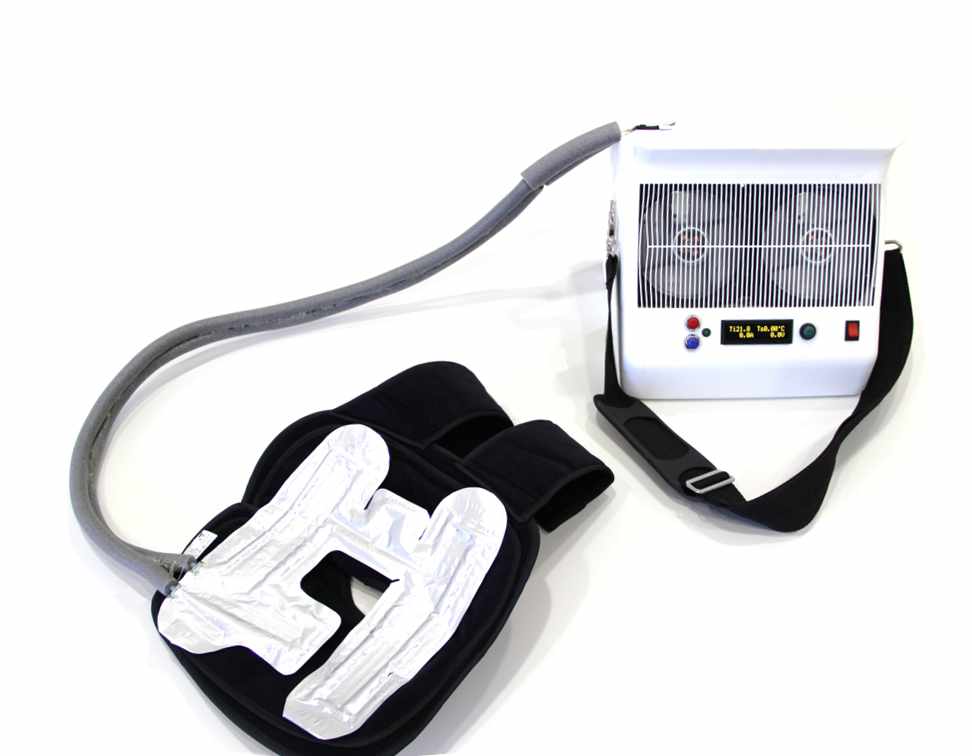
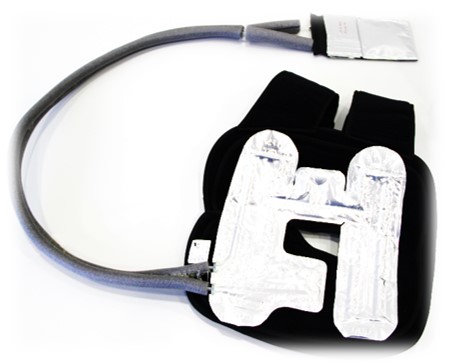
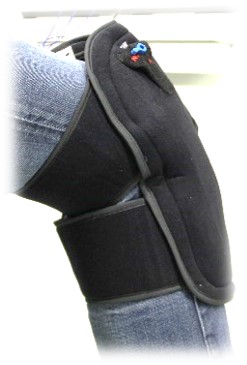
Thermotherapy provides significant benefits in many applications by yielding pain relief and de-swelling. Patients will require less pain medication. The precise temperature control guarantees optimal healing progress and prevents tissue damages due to frostbite. The desirable mobilization or patients in rehabilitation is supported through the low weight of the device enabling mobile use outside of clinic and practice.
Everything started when our former colleague, Prof. Dr. Jürgen Mollenhauer (†) complained about his swollen, aching ankle, which had been fractured months before and had had surgery, yet still wasn’t healing. “If only there were a cooling device to reduce pain and swelling, which wasn’t so ice cold…” So, what temperature should it be? Surprisingly, 10 – 20°C was the answer.
A few hours later, one of the founders put a cooling element on Prof. Mollenhauers table, built from spare parts in the lab: a peltier device with fan and copper sheet to draw body heat. Surprised by Jürgen’s euphoric response we thought: “if this meek prototype is received so well, we might as well develop this into a medical device business." mollicool was born.

Initial market research on thermotherapy during the MedTech Startup School led to the formation of the founder team, Stefan Thiele and Dr. Martin Stelzle, a first patent application and to the decision to further develop this technology into a medical device.

Thermal and fluidic multiphysics simulation, extensive research of materials and production technologies yield a first fully functional prototype with convincing cooling and heating performance.


The mollicool team consists of experts in their respective field including business development as well as medical device engineering. Together with our advisory board we strive for products that will help our patients to get well soon and provide them with a superior user experience while using mollicool.

Business development

Technical development

NMI lab director
Medical technology is one of the core competences of the NMI and supporting the creation of successful high tech startups is at the heart of NMI’s mission. We are therefore delighted to support the MOLLICool team in its development of a truly innovative medical device.

deputy lab director, entrepreneur, mentor of mollicool team
As founder of my own company I am well aware of the hurdles on the way to creating a business. It is my great pleasure to advice such an enthusiastic and competent team on their way to making mollicool successful.

MEDTECH360, Advisor Regulatory Affairs
As a founder of a medical device spin-off myself, I am pleased to advise and guide the mollicool team in the establishment of the complex technical documentation required to enable timely and successful market entrance.

stimOS GmbH, Regulatory Affairs, 13485 Certification
The novel medical device regulations are extremely demanding for a startup such as mollicool on their way to gain certification of their enterprise and approval for their product. As the mollicool device relates to numerous norms requiring a tailored QM system, we provide competent support for mollicool on their way to the market for medical devices.
The NMI has an impressive track record as incubator for high tech startups with 15 spin-offs founded so far during its 35 years of activity. The NMI hosts the mollicool team in its incubator and provides comprehensive technical and administrative support.
Are you interested in our products or do you want to ask another question?
Then please fill out the following contact form: